Your patient has been diagnosed with an aggressive form of cancer.
A personalized vaccine could be the answer.
The Jaime Leandro Foundation can help.
Personalized Neoantigen Vaccines through Expanded Access
Traditional vaccines revolutionized the treatment of disease. In recent years, personalized vaccines have shown promise in combating cancer. In addition, technological advances in genetic sequencing, algorithms to identify neoantigens, and peptide manufacturing have vastly improved the personalized vaccine development process.
Of critical importance, the FDA “Expanded Access” rule gives an individual the right to try a medical treatment when no comparable alternative options are available.
Turn to JLF
The Jaime Leandro Foundation (JLF) is a private nonprofit organization established to deliver breakthrough cancer treatments based on the latest innovations in research and personalized medicine through clinical trials and by leveraging the Expanded Access rule. We bring the critical components together, partnering with expert academic and commercial institutions to develop and deliver therapeutic vaccines personalized for each patient.
In essence, JLF is the hub, and our partners use advanced genetic sequencing, identify the best patient-specific neoantigen targets, and manufacture, assemble, vial, and ship the personalized vaccine, which is administered by the treating physician. Then, JLF partners monitor and report results for the advancement of the field. Learn more.
Collaborators
Our collaborators are at the forefront of medicine and science. Each is an industry leader providing cutting-edge expertise and technology. JLF consults with the patient, coordinates with partners, and helps both navigate the entire process.

Taking the work out of paperwork
We know: preparing and submitting the paperwork necessary for Expanded Access can be a nuisance, taking you away from the many pressing demands on your time. That’s why JLF will assist with preparation of the FDA Expanded Access application. Upon approval, the FDA issues the patient an Investigational New Drug (IND) ID, which facilitates shipment of the vaccine.
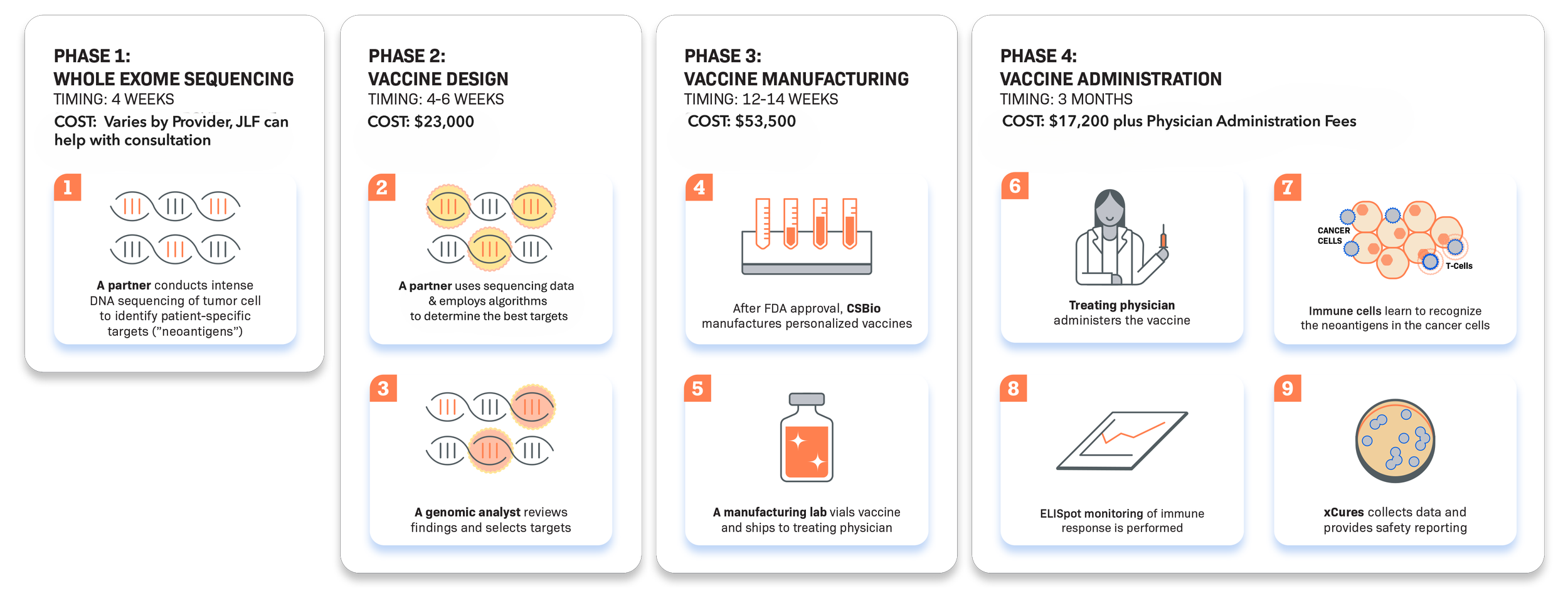
The JLF Process
The JLF process involves industry leaders providing cutting-edge technology at every step. JLF consults and contracts with the patient to discuss costs, holds IND and trial liability insurance, and guides the entire process. Our partners manage vaccine design, vaccine manufacturing, and patient protocol startup and consent with the treating physician. Developing a personalized therapeutic vaccine takes 4-5 months.
For more information on how to partner with JLF, please contact us.